Highly active cobalt oxidecatalysts by in situ oxidation of carbon-encapsulated cobalt in hydrocarbon combustion |
|
Combustion catalysts have been extensively explored to reduce the emission of hydrocarbonsthat are capable of triggering photochemical smog and greenhouse effect. Palladium as themost active material is widely applied in exhaust catalytic converter and combustion units,but its high capital cost stimulates the tremendous research on non-noble metal candidates.Extensive research has been performed in searchingcandidates for noble metal catalysts, but there is still alarge demand for practically useful and acceptable catalystsfor a variety of important reactions. As a cheap candidate to replace noble metals forcatalytic combustion, Co3O4 nanoparticles show a pronouncedparticle size effect on the reaction pathway of cyclohexaneconversion. Smaller nanoparticles with more edge and cornersites would lead to more strongly bound oxygen species tomediate the combustion pathway rather than oxidativedehydrogenation one.
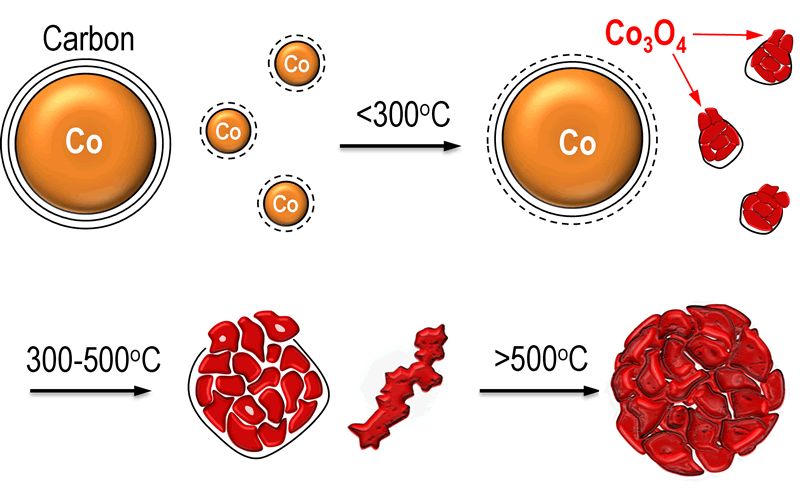
Fig. Scheme for the transformation process of the Co3O4 catalyst.
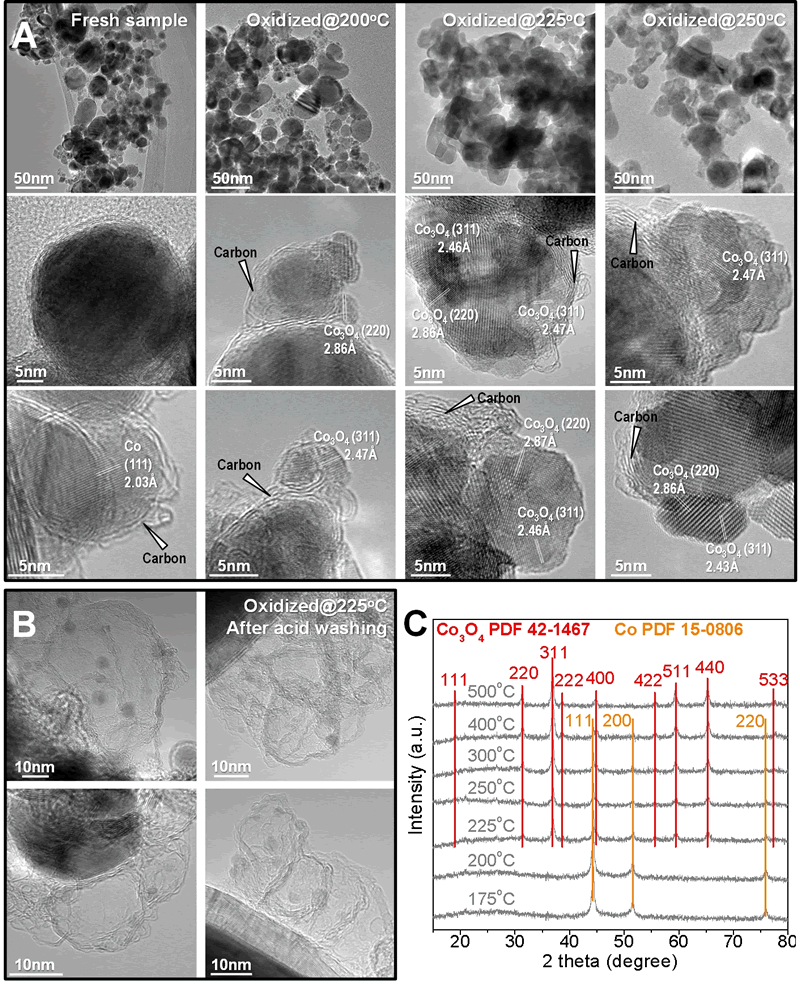
Fig. Morphological properties of oxidized Co@C nanocapsules. (A) TEM and HRTEM images of the pristine and samples that were oxidized at 200-250 oC. (B) HRTEM images of the residual carbon shells in the oxidized samples at 225¡æafter washing by hydrochloric acid. (C) XRD spectra of oxidized samples.
|
|